n Jan. 5, 2020, the World Health Organization first reported that a “pneumonia of unknown cause” had emerged in China. At first, Americans took little notice — except for a few leading medical institutions that included the University of Maryland School of Medicine (UMSOM), where faculty physicians and scientists immediately began to mobilize. By early February, several research initiatives at the school were underway, attracting national coverage by The Washington Post.
Six months later, UMSOM remains a national leader in our country’s effort to combat the COVID-19 global pandemic, with experts across its academic departments, research centers, and institutes actively engaged in myriad initiatives. These medical and scientific leaders have been on the front lines in studying the virus, developing innovative testing programs, investigating potential vaccines, and exploring a range of novel therapies. Many of these UMSOM faculty experts have appeared regularly in national and international news media to discuss initiatives related to testing, treatment, and vaccine development.
Wilbur Chen, MD, associate professor of medicine and an adult infectious disease specialist, and David Marcozzi, MD, MHS-CL, FACEP, associate professor of emergency medicine and head of the Incident Command for the University of Maryland Medical System, were tapped to serve on Maryland Gov. Larry Hogan’s COVID-19 response task force, where they continue to advise top state officials on the illness, treatment, and containment.
“We are witnessing historic efforts by medical schools around the world to develop and manufacture diagnostics, treatment, and vaccines in record time,” notes UMSOM Dean E.Albert Reece, MD, PhD, MBA, who also is executive vice president for medical affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, UMSOM. “At UMSOM, we are leading efforts — nationally and internationally — to combat this pandemic across multiple fronts, with research and discovery at the core of these efforts. We are seeing unprecedented innovation and collaboration amongst our faculty, with more than 100 individual COVID-19-related research projects underway.”
In a significant paper published in the journal JAMA Network Open, researchers from UMSOM’s Institute of Human Virology (IHV) found that all cities experiencing significant outbreaks of COVID-19 had very similar winter climates with an average temperature of 41 to 52 degrees Fahrenheit, an average humidity level of 47 to 79 percent with a narrow east-west distribution along the same 30 to 50 degrees north latitude. They predict that COVID-19 will follow a seasonal pattern similar to other respiratory viruses like seasonal flu. They based this on weather modeling data in countries where the virus had taken hold and spread within the community.
“Based on what we have documented so far, it appears that the virus has a harder time spreading between people in warmer, tropical climates,” said study leader Mohammad Sajadi, MD, associate professor of medicine, UMSOM, physician-scientist at IHV, and a member of the Global Virus Network. He is working with colleagues to launch a real-time forecasting model of climate conditions that are favorable to the spread of COVID-19.
UMSOM researchers also have helped to validate several human antibodies that can be mixed in a “cocktail” and used as a promising antiviral therapy against COVID-19. Their research, conducted in collaboration with scientists at Regeneron Pharmaceuticals, was published June 15 in the journal Science. It demonstrates the rapid process of isolating, testing, and mass-producing antibody therapies against any infectious disease by using genetically engineered mice as well as plasma from recovered COVID-19 patients. The antibody cocktail tested in this study will be used to treat COVID-19 patients in a clinical trial that Regeneron launched in June.
UMSOM researchers are the first in the United States to begin testing a new experimental COVID-19 vaccine candidate. At present, there are no licensed vaccines or therapies for COVID-19. The BNT162 Program is a collection of at least four experimental vaccines, each of which represents a different combination of mRNA formats and target protein antigens, which stimulate the immune system of the vaccinated individual. The vaccine research is part of a multicenter study in the United States and Germany.
Kirsten Lyke, MD, professor of medicine, is a lead investigator in this research, along with Kathleen Neuzil, MD, MPH, the Myron M. Levine, MD, DTPH, Professor in Vaccinology, professor of medicine and pediatrics, and director of UMSOM’s Center for Vaccine Development and Global Health (CVD). “We are excited to begin testing these vaccine candidates against COVID-19. This research is on a fast track, given the extreme consequences of this pandemic and the critical need for preventive measures,” Neuzil said.
In July, CVD researchers will begin a major trial of another mRNA vaccine candidate developed by Moderna, a clinical-stage biotechnology company. Following Phase 1 trials for this vaccine in March, CVD will now oversee this next critical phase of testing, which will help pave the way to licensure. Volunteer recruitment for this vaccine study will focus on under-represented populations that have been impacted more heavily by the COVID-19 pandemic, including African American and Latin communities.
In addition to testing vaccines and COVID-19 therapies, CVD researchers have served as a critical resource for pediatricians and other child care providers.
CVD experts launched a COVID-19 website for pediatric health care providers and practices, parents, and children. This resource brings together important research, professional guidance for pediatric practices, and practical tips for parents and caregivers. The site includes resources for pediatric practices and hospitals including protocols, guidance, and clinical resources for COVID-19.
Treatment
UMSOM researchers have begun testing an experimental stem cell therapy developed by Mesoblast Limited to treat hospitalized COVID-19 patients with moderate to severe acute respiratory distress syndrome (ARDS) who are on ventilators to help them breathe. The trial, which is being conducted at the University of Maryland Medical Center (UMMC) and additional sites across the country, will involve 300 patients randomized to receive the drug Remestemcel-L or a placebo in addition to the recommended standard of care to manage severe COVID-19 infections. The first patient in this national trial was treated at UMMC.
The research, funded by Mesoblast, an Australian regenerative medicine company, is designed to determine whether the drug reduces the risk of death within 30 days after the onset of treatment and whether it reduces the number of days from a ventilator to recovery.
Researchers at CVD are testing whether hydroxychloroquine can be used to help prevent COVID-19 illness in individuals who have been exposed to this illness. The research is critical, as more than three-quarters of COVID-19 transmissions occur through close contact within households. The trial includes volunteers in several states and the District of Columbia. Hydroxychloroquine is a drug in the same family of therapies used to treat malaria, enabling CVD’s malaria experts to quickly pivot research toward COVID-19. The trial is significant as it focuses on preventing COVID-19 and does not involve individuals who are ill with infection but rather healthy individuals who have been exposed.
In March, CVD researchers began testing the antiviral drug remdesivir on hospitalized COVID-19 patients. This National Institutes of Health-funded research, led by Karen Kotloff, MD, professor of pediatrics, and Justin Ortiz, MD, associate professor of medicine, is part of a larger nationwide study, with early results showing some positive effects in reducing the duration of illness and mortality in severely ill patients. The Food and Drug Administration (FDA) has now approved the use of remdesivir to treat COVID-19 in severely ill hospitalized patients.
“This trial brings opportunities for our patients at the University of Maryland Medical Center to receive the drug under carefully controlled conditions and provide the critical data needed for licensure of remdesivir as a COVID-19 treatment by the FDA,” said Kotloff, who is the associate director for clinical research at CVD.

Neal Reynolds, MD, associate professor of medicine, treats patients with severe COVID-19 at the R Adams Cowley Shock Trauma Center — all while sitting at his desk at home some 22 miles away. By remotely operating “Fast Freddy,” one of six high-tech robots operating in Shock Trauma’s COVID-19 units, Reynolds can conduct a complete exam and communicate directly with patients without risk of becoming infected himself. The use of these advanced robots protects health care workers and helps prevent the spread of the virus. Through the use of “Fast Freddy,” Reynolds can monitor vital signs, access patient records, and consult with colleagues as the semi-autonomous robot makes its rounds.
Access (testing and community engagement related to COVID-19)

UMSOM has launched a large-scale COVID-19 testing initiative that will be progressively ramped up to eventually run as many as 20,000 tests per day. The initiative is funded by $2.5 million from the state of Maryland and will allow for far wider access to testing in Maryland through coordination with the city of Baltimore and the state Department of Health. The testing facility at UMSOM would be able to return the results to patients and doctors within 24 to 48 hours, dramatically increasing the turnaround time.
Test samples are being processed on robotic platforms with automated technologies housed in a laboratory at the Institute for Genome Sciences at UMSOM. State funding will allow for the purchase of additional platforms to facilitate an increase in testing capacity.
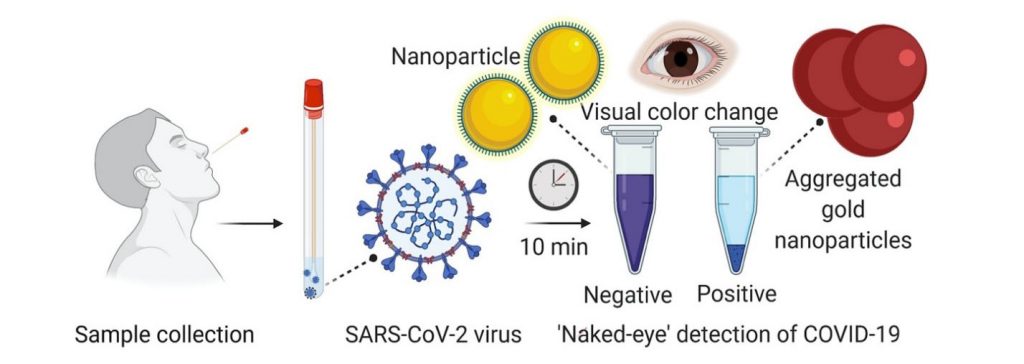
UMSOM scientists also have developed a highly reliable diagnostic test for COVID-19 that allows anyone to visually detect the presence of the virus in 10 minutes. The test does not require the use of any advanced laboratory techniques for analysis.
“This new test may detect RNA material from the virus as early as the first day of infection, making it superior to other rapid COVID-19 tests, which often cannot detect the virus until several days after infection,” said study leader Dipanjan Pan, MSc, PhD, UMSOM professor of diagnostic radiology and nuclear medicine and pediatrics. “For this reason, many of the diagnostic tests currently on the market detect virus antibodies and have a higher rate of false negative results if they are performed early in the infection.”
The University of Maryland Addiction Treatment Center, at 1001 W. Pratt St. in Baltimore, is the first hospital-based Medication-Assisted Treatment program in Maryland. The center’s Health and Recovery Practice (HARP) is working to serve the needs of its homeless patients during the pandemic, providing hand sanitizer, soap, wipes, tissues, alcohol wipes, and bottled water. HARP also has introduced telehealth capabilities, increasing the number of patients it can see every week, while connecting patients with housing at motels and residential treatment centers, and with family members.